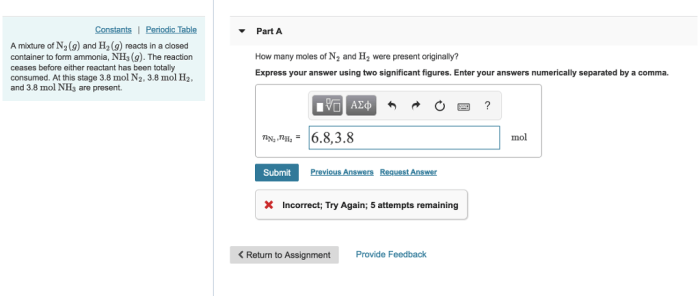
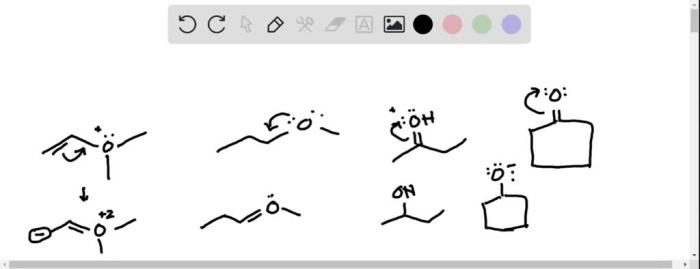
In the realm of chemistry, curved arrows play a crucial role in depicting the movement of electrons during chemical reactions. However, there are specific rules that govern their usage. Which of the following violates the rules for curved arrows? This article delves into the fundamental principles of curved arrows, their relationship with electron movement, and explores exceptions to the typical conventions.
By understanding these rules and their limitations, chemists can accurately represent reaction mechanisms and gain a deeper comprehension of chemical processes.
The intricacies of curved arrow notation extend beyond the basic rules. In certain scenarios, exceptions arise, challenging the conventional application of curved arrows. This article will shed light on these exceptions, providing examples of reactions that defy the typical guidelines.
Additionally, the use of curved arrows in resonance structures and organic reactions will be examined, highlighting their significance in understanding the stepwise progression of complex chemical transformations.
Basic Rules of Curved Arrows
Curved arrows in chemical reactions are a graphical representation of electron movement. They follow specific rules to accurately depict the changes in electron distribution during a reaction.
Rules:
- Electron Flow:Curved arrows always show the movement of electrons.
- Head and Tail:The head of the arrow points to the electron acceptor (positive charge or electronegative atom), while the tail points to the electron donor (negative charge or electropositive atom).
- Conservation of Charge:The total number of electrons in the reactants and products must be the same.
Invalid Curved Arrow Notations:
- Arrows that start or end in empty space.
- Arrows that point in the wrong direction.
- Arrows that violate the conservation of charge.
Electron Movement and Curved Arrows
Curved arrows depict the flow of electrons in reactions. The direction of the arrow indicates the movement of the electron pair from the electron donor to the electron acceptor.
Example:
- In the reaction HCl + NaOH → NaCl + H 2O, the curved arrow from the lone pair of electrons on OH –to the H +ion represents the transfer of electrons to form H 2O.
Curved arrows help visualize the electron movement and understand the reaction mechanism.
Exceptions to Curved Arrow Rules
In certain scenarios, curved arrow rules may not apply strictly.
- Concerted Reactions:In concerted reactions, electron movement occurs simultaneously, making it difficult to depict using curved arrows.
- Radical Reactions:Radicals have unpaired electrons, which can lead to unconventional electron movement not always captured by curved arrows.
- Hypervalent Compounds:Compounds with atoms exceeding their typical valence electron count may require adjustments to curved arrow notation.
Curved Arrows in Resonance Structures
Curved arrows can be used to represent resonance structures. They show the movement of electrons between different resonance contributors.
Example:
- In the resonance structures of benzene, the curved arrows indicate the movement of electrons within the ring, leading to the delocalization of electrons.
Curved arrows help visualize the electron distribution and understand the resonance phenomenon.
Curved Arrows in Organic Reactions: Which Of The Following Violates The Rules For Curved Arrows
Curved arrows play a crucial role in illustrating reaction mechanisms in organic chemistry.
- Nucleophilic Substitution:Curved arrows show the attack of a nucleophile on an electrophile, leading to the formation of a new bond.
- Electrophilic Addition:Curved arrows depict the addition of an electrophile to a double or triple bond, forming new bonds.
- Elimination Reactions:Curved arrows show the removal of a leaving group and the formation of a new double or triple bond.
Curved arrows help visualize the stepwise progression of organic reactions and understand their mechanisms.
Questions and Answers
What is the primary purpose of curved arrows in chemical reactions?
Curved arrows serve as a visual representation of electron movement, indicating the flow of electrons from one atom or molecule to another during a chemical reaction.
Can curved arrows be used to depict the movement of any type of particle?
No, curved arrows are specifically used to represent the movement of electrons. The movement of other particles, such as protons or neutrons, is not typically depicted using curved arrows.
Are there any exceptions to the rules governing curved arrow notation?
Yes, there are certain scenarios where the typical rules for curved arrows may not apply. These exceptions will be discussed in detail in the article.