Embark on an exploration of intermolecular forces with our comprehensive Chemistry Unit 4 Compounds Intermolecular Forces Worksheet. This resource delves into the intricacies of intermolecular interactions, providing a solid foundation for understanding the physical properties and behavior of compounds.
Our worksheet begins by introducing the concept of intermolecular forces, exploring their types and the factors that influence their strength. We then delve into the relationship between intermolecular forces and the physical properties of compounds, examining how these forces impact melting point, boiling point, and solubility.
Through real-world examples, we illustrate the significance of intermolecular forces in everyday life, industry, and biological systems.
Intermolecular Forces: Chemistry Unit 4 Compounds Intermolecular Forces Worksheet
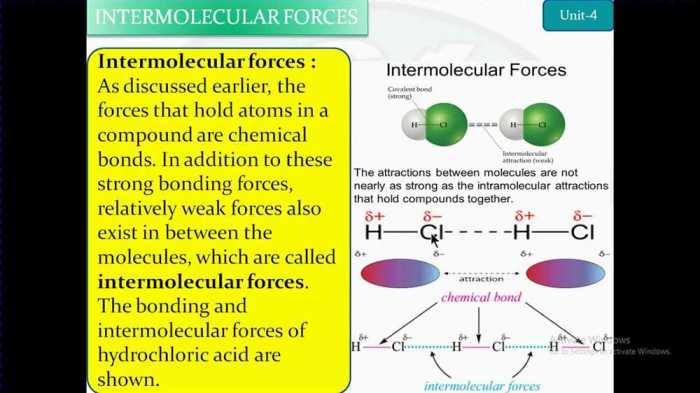
Intermolecular forces (IMFs) are the attractive forces that act between molecules. They are weaker than the intramolecular forces that hold atoms together within a molecule. IMFs determine many of the physical properties of compounds, such as melting point, boiling point, and solubility.
There are three main types of IMFs: dipole-dipole forces, hydrogen bonding, and van der Waals forces. Dipole-dipole forces occur between polar molecules that have a permanent dipole moment. Hydrogen bonding is a special type of dipole-dipole force that occurs between molecules that have a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine.
Van der Waals forces are the weakest type of IMF and occur between all molecules, regardless of their polarity.
The strength of IMFs depends on several factors, including the polarity of the molecules, the size of the molecules, and the shape of the molecules. Polar molecules have stronger IMFs than nonpolar molecules. Larger molecules have stronger IMFs than smaller molecules.
Molecules with more complex shapes have stronger IMFs than molecules with simpler shapes.
Properties of Compounds
IMFs have a significant impact on the physical properties of compounds. Compounds with strong IMFs have higher melting points and boiling points than compounds with weak IMFs. This is because it takes more energy to overcome the IMFs and separate the molecules in a compound with strong IMFs.
IMFs also affect the solubility of compounds. Compounds with strong IMFs are less soluble in nonpolar solvents than compounds with weak IMFs. This is because the IMFs between the molecules of the compound are stronger than the IMFs between the molecules of the compound and the molecules of the solvent.
The following table shows the melting points, boiling points, and solubilities of some common compounds with different types of IMFs:
| Compound | Type of IMF | Melting point (°C) | Boiling point (°C) | Solubility in water (g/100 mL) |
|---|---|---|---|---|
| Methane | Van der Waals forces | -182.5 | -161.6 | 0.001 |
| Ethanol | Hydrogen bonding | -114.1 | 78.3 | Infinite |
| Sodium chloride | Ionic bonding | 801 | 1413 | 35.7 |
Types of Compounds
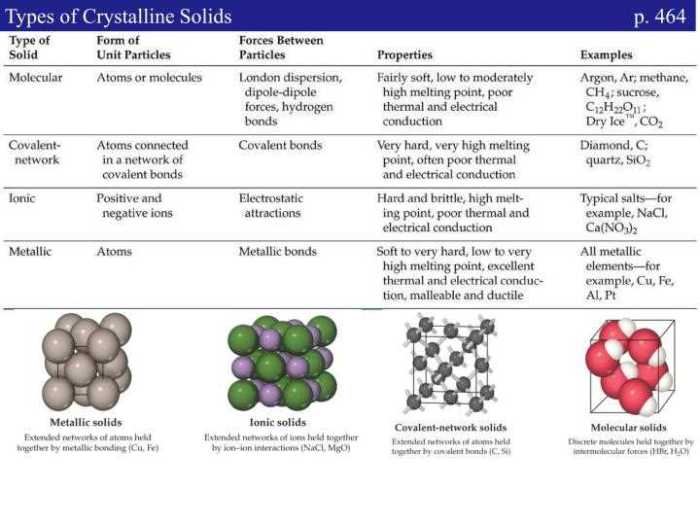
Compounds can be classified into different types based on the type of IMFs that they have. The three main types of compounds are:
- Nonpolar compounds: These compounds have no permanent dipole moment and only have van der Waals forces. Examples of nonpolar compounds include methane, ethane, and propane.
- Polar compounds: These compounds have a permanent dipole moment and have dipole-dipole forces. Examples of polar compounds include ethanol, water, and ammonia.
- Ionic compounds: These compounds are formed by the transfer of electrons from one atom to another. Ionic compounds have strong electrostatic forces between the ions. Examples of ionic compounds include sodium chloride, potassium chloride, and calcium oxide.
Intermolecular Forces in Real-World Applications
IMFs play an important role in many everyday applications. For example, IMFs are responsible for the adhesion of materials, such as glue and tape. IMFs are also responsible for the surface tension of liquids, which allows insects to walk on water.
In the biological world, IMFs are responsible for the structure of DNA and proteins.
The following are some specific examples of how IMFs are used in various industries:
- Adhesives: IMFs are responsible for the adhesion of materials, such as glue and tape. The stronger the IMFs between the adhesive and the surface, the stronger the bond will be.
- Paints and coatings: IMFs are responsible for the adhesion of paints and coatings to surfaces. The stronger the IMFs between the paint or coating and the surface, the more durable the finish will be.
- Pharmaceuticals: IMFs are responsible for the solubility of drugs in water. The solubility of a drug is important for its absorption and distribution in the body.
- Food science: IMFs are responsible for the texture of foods. For example, the strong IMFs between water molecules are responsible for the high surface tension of water, which gives water its “wet” feel.
Q&A
What are intermolecular forces?
Intermolecular forces are attractive or repulsive forces that act between molecules or atoms.
What are the different types of intermolecular forces?
The three main types of intermolecular forces are dipole-dipole forces, hydrogen bonding, and van der Waals forces.
How do intermolecular forces affect the physical properties of compounds?
Intermolecular forces influence properties such as melting point, boiling point, and solubility by affecting the strength of the interactions between molecules.