Which statements about peptide bonds are true – Exploring the realm of peptide bonds, this discourse delves into their multifaceted nature, unraveling the intricacies of their formation, properties, and cleavage. This comprehensive examination unveils the significance of peptide bonds in shaping the structure and function of proteins, providing a deeper understanding of their fundamental role in biological processes.
Types of Peptide Bonds
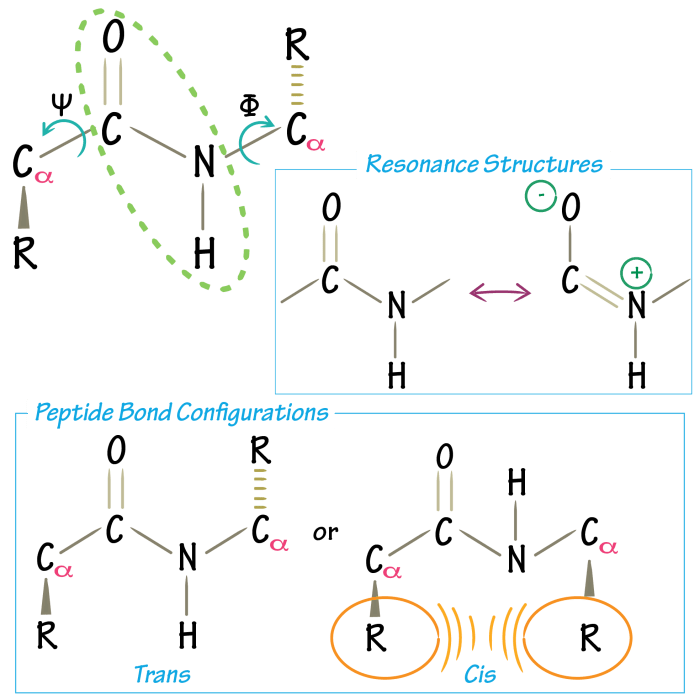
Peptide bonds are classified based on the orientation of the amide group’s carbonyl oxygen and nitrogen atoms relative to the alpha-carbon of the amino acid residue. The two main types of peptide bonds are:
- Cis peptide bond:The carbonyl oxygen and nitrogen atoms are on the same side of the alpha-carbon.
- Trans peptide bond:The carbonyl oxygen and nitrogen atoms are on opposite sides of the alpha-carbon.
The trans peptide bond is the predominant form found in proteins due to steric hindrance and the formation of more stable hydrogen bonds.
Formation of Peptide Bonds
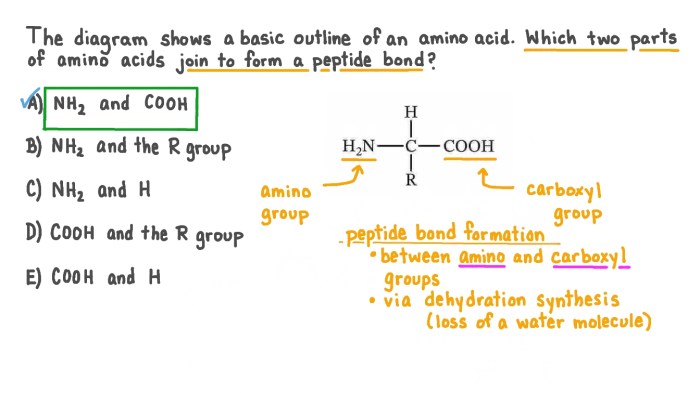
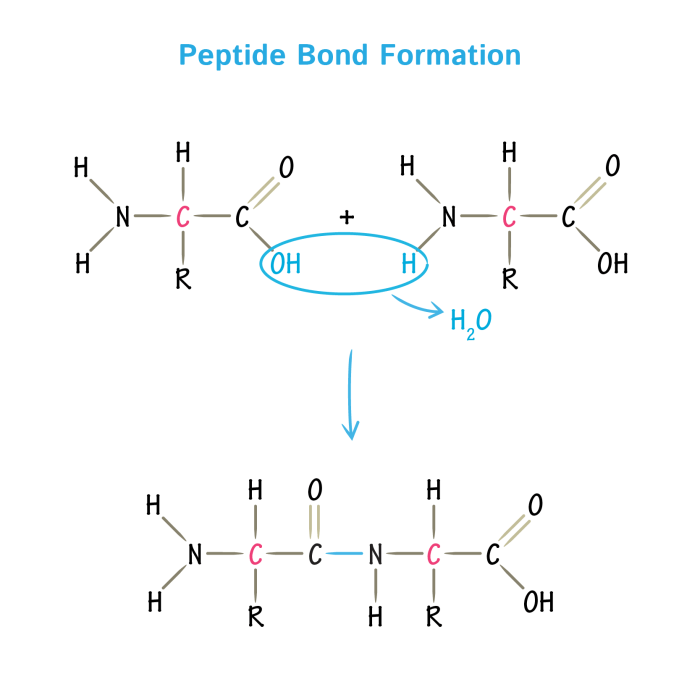
Peptide bonds are formed through a condensation reaction between the carboxyl group of one amino acid and the amino group of another amino acid.
The reaction proceeds in two steps:
- Nucleophilic attack:The amino group of one amino acid attacks the carbonyl carbon of the other amino acid, forming a tetrahedral intermediate.
- Proton transfer:A proton is transferred from the tetrahedral intermediate to a base, forming the peptide bond and releasing water.
The formation of peptide bonds is catalyzed by enzymes called peptidases.
Properties of Peptide Bonds
Peptide bonds are strong covalent bonds with the following properties:
- Partial double bond character:The resonance between the carbonyl oxygen and nitrogen atoms gives the peptide bond partial double bond character, restricting rotation around the bond.
- Polarity:The peptide bond is polar due to the electronegativity difference between the carbonyl oxygen and nitrogen atoms.
- Hydrogen bonding:The carbonyl oxygen and nitrogen atoms can participate in hydrogen bonding, which stabilizes the peptide bond and contributes to protein structure.
Cleavage of Peptide Bonds
Peptide bonds can be cleaved through hydrolysis, which is the reverse of the peptide bond formation reaction.
Hydrolysis of peptide bonds can occur:
- Spontaneously:Peptide bonds can hydrolyze slowly in water, but the rate is negligible at physiological pH.
- Enzymatically:Enzymes called proteases specifically cleave peptide bonds.
The factors that affect peptide bond cleavage include pH, temperature, and the presence of proteases.
FAQ Corner: Which Statements About Peptide Bonds Are True
What is the primary function of peptide bonds?
Peptide bonds serve as the covalent linkages between amino acids, forming the backbone of proteins. They determine the sequence and connectivity of amino acids, shaping the overall structure and function of proteins.
How are peptide bonds formed?
Peptide bond formation occurs through a condensation reaction between the carboxyl group of one amino acid and the amino group of another, releasing a molecule of water. This process is catalyzed by enzymes known as peptidases.
What factors influence peptide bond cleavage?
Peptide bond cleavage is influenced by various factors, including pH, temperature, and the presence of specific enzymes called proteases. These enzymes hydrolyze peptide bonds, breaking down proteins into smaller fragments.